
“Now we are still trying to attract industrial partners to fund us to characterize the process in more detail and to determine exactly how much nanodiamond needs to be added to the electrolyte in particular applications.”...

Nanodiamonds in the electrolyte solution of lithium ion batteries prevent the formation of dendrites that cause runaway heat-up resulting in fires.
Source: Drexel University and Tsinghua University
Scientists researching battery-related fires and explosions, such as the incidents that got Samsung Galaxy Note 7s banned from airline flights last year, took the first step toward preventing the fires when they traced the source of the runaway heat buildup to small dendrites that form between the anode and cathode. Now a materials specialist at Drexel University (Philadelphia) has proposed a low-cost, easy way to prevent the dendrites from forming: Just mix in nanodiamonds with the ordinary lithium-ion electrolyte at a concentration of at least 1 percent.
Drexel professor Yury Gogotsi, working with a doctoral candidate visiting from Tsinghua University (Beijing), discovered the method. But getting Samsung and the myriad other Li-ion battery producers and OEMs to sign on to the concept has proved more difficult than confirming that the nanodiamond additive works.
“We had to use internal funding from Drexel to even prove the concept,” Gogotsi told EE Times. “Now we are still trying to attract industrial partners to fund us to characterize the process in more detail and to determine exactly how much nanodiamond needs to be added to the electrolyte in particular applications.”
It’s possible that the “diamond” in nanodiamond is putting off cost-conscious manufacturers, as Li-ion battery technology already is expensive. But that concern is unfounded, Gogotsi said, since nanodiamonds are cheap to manufacture and, in fact, can be created from waste materials.
“All you need to do is take expired explosives, which are otherwise expensive to dispose of, and explode them in a sealed chamber,” Gogotsi said. “The result will be a coating on the walls of the chamber that is more than 50 percent nanodiamonds typically measuring just 5 nanometers across.”
The mechanism, believe it or not, is analogous to the way Superman made diamonds in the comic books: The superhero applied incredibly high pressure to ordinary carbon, forcing it into its most compact structure. Of course, the Man of Steel used his bare hands, whereas Gogotsi’s method depends on the incredible pressures created by an explosion in a closed space.
Gogotsi’s lab uses but “did not create” the process for creating nanodiamonds, he said. “In fact, it was invented by three separate laboratories in Russia and was kept so secret that each lab was unaware of the other labs’ similar discovery.”
Los Alamos National Lab eventually published a description of the process, which today is used worldwide to turn hard-to-dispose-of waste — such as expired C4 — into marketable products. Nanodiamonds are widely used today in such products as industrial abrasives, medical coatings, and electronic sensors that measure magnetic fields.
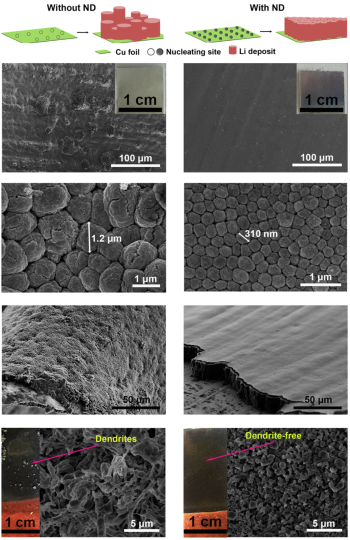
Then progressive growth of dendrites (left) in electrolytes without at least 1 percent nanodiamond, compared to the dendrite-free use of Li-Ion batteries with nano diamonds (right).
Source: Drexel University
Now nanodiamonds are poised to solve the igniting-battery problem that killed off the Galaxy Note 7 — if manufacturers can be convinced to use them.